Immune cells in the human brain may be critical to orchestrating the organ’s development in the womb because they trigger a dramatic increase in an important type of nerve cell, new research suggests.
Estimates suggest that these key cells, known as inhibitory interneurons, make up some 25% to 50% of the neurons in the adult cortex, the wrinkled tissue that covers the surface of the brain. In fact, the human cortex carries more than double the number of interneurons as the mouse cortex does.
These interneurons relay signals between other brain cells and help keep that signaling in check with a chemical messenger called GABA. As the brain’s main “inhibitory” messenger, GABA helps turn down brain activity by making neurons less likely to fire, thus balancing out the “excitatory” signals that amplify brain activity. Various disorders have been tied to problems with interneurons, including epilepsy, autism and schizophrenia.

Now, in a study published Aug. 6 in the journal Nature, researchers have uncovered a force that drives interneurons to multiply in the developing human brain — and they say it may be unique to our species.
“That’s why we cannot use traditional animal models,” study co-author Diankun Yu, an assistant researcher in pediatrics at the University of California, San Francisco (UCSF), told Live Science. To uncover this mechanism that may unfold only in the human brain, the researchers developed an organoid — a miniature 3D structure, grown from stem cells, that mimics a full-size structure found in the human body.
Prior to the organoid study, research in lab animals suggested a link between the activation of the maternal immune system during pregnancy and a lower number of interneurons in the cortices of their offspring, compared to offspring that didn’t experience an immune upset. That kind of activation might occur in response to a viral or bacterial infection, for example. The study authors explored this in previous research with lab mice, in which they pinpointed a key player behind the link: microglia, the brain’s resident immune cells.
Related: In a 1st, scientists combine AI with a ‘minibrain’ to make hybrid computer
In the past five years, scientists have begun to recognize how the immune system and nervous system develop in parallel, study co-author Dr. Xianhua Piao, a physician-scientist who specializes in neonatology and developmental neuroscience at UCSF, told Live Science.
“The microglia really fine-tune and regulate nervous development,” she said of the new study’s findings. “It really adds a new dimension as to how microglia exert their function.”
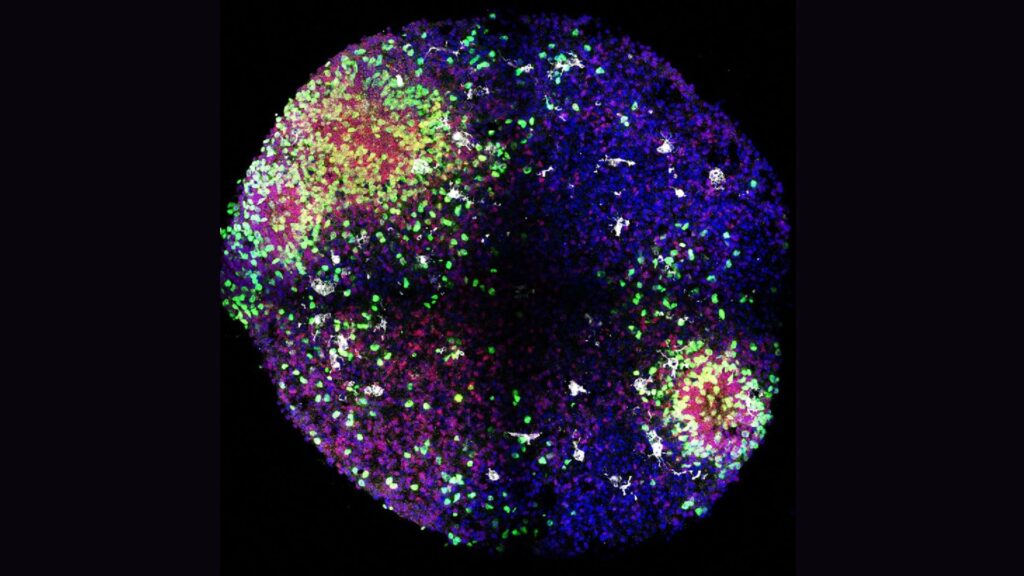
The team built on previous work from other research groups to develop their organoids, which resembled a key structure in the fetal brain from which many cortical interneurons arise. This structure is temporary, appearing around the eighth week of pregnancy in humans and disappearing about eight months after birth, Piao said. The researchers found a way to incorporate microglia into this model, which hadn’t been done before, she added.
The team found that the microglia in their organoids were a key source of insulin-like growth factor 1 (IGF1) in the developing minibrains and that the substance helped to drive the dramatic increase in interneurons seen in early development.
When the team tested what would happen when they turned off IGF1 signaling in various ways, they found that it blocked the rapid increase in interneurons. However, “when we deleted this gene in microglia in the mouse model, we did not see any change,” Piao said. That suggests that this chain of events kicked off by microglia-made IGF1 may be unique to humans.
“These findings indicate an evolutionary adaptation of microglial function to support the increased demand for interneurons in the human cortex,” the researchers wrote in their report. In short, this finding hints at a feature of human evolution that might help to explain our unique cognitive abilities.
That said, organoids aren’t exact replicas of the human brain, so there’s a limit to what the 3D models can tell us. “So far the model is good enough for especially the proliferation stage, very early stage” of development, Yu said. But currently, these organoids don’t do as well with later stages of brain development, he noted. They also don’t capture circuit-level activity in the brain, Piao said, showing only activity within smaller, isolated structures.
Future work could help to further clarify this previously unknown role of immune cells in the brain, she said.