If bacteria had a list of things to fear, phages would be at the top. These viruses are built to find, infect and kill them — and they have been doing it for billions of years. Now that ancient battle is offering clues for how we might fight back against antibiotic-resistant infections.
As more bacteria evolve to withstand our antibiotics, previously treatable infections are becoming harder — and in some cases, impossible — to cure. This crisis, known as antimicrobial resistance (AMR), already causes over a million deaths a year globally, and the number is rising fast. The World Health Organization has named AMR one of the top ten global public health threats.
Phage therapy — the use of phages to treat bacterial infections — is gaining attention as a potential solution. Phages are highly specific, capable of targeting even drug-resistant strains. In some compassionate-use cases in the U.K., they have cleared infections where every antibiotic had failed. But phages still face a challenge that is often overlooked: the bacteria themselves.
Bacteria have evolved sophisticated systems to detect and destroy phages. These defenses are diverse: some cut up viral DNA, others block entry, and a few launch a kind of intracellular shutdown to prevent viral takeover. In a new study published in Cell, my colleagues and I describe a system that works differently, called Kiwa. It acts like a sensor embedded in the bacterial membrane, detecting early signs of attack.
Exactly what Kiwa is sensing remains an open question, but our findings suggest it responds to the mechanical stress that occurs when a phage latches on to the cell and injects its DNA. Once triggered, Kiwa acts fast. It shuts down the phage’s ability to make the components it needs to build new phages, stopping the infection before it can take over the cell.
But just as bacteria evolve ways to defend themselves, phages evolve ways to fight back. In our latest experiments, we saw two strategies in play.
Related: ‘Medicine needed an alternative’: How the ‘phage whisperer’ aims to replace antibiotics with viruses
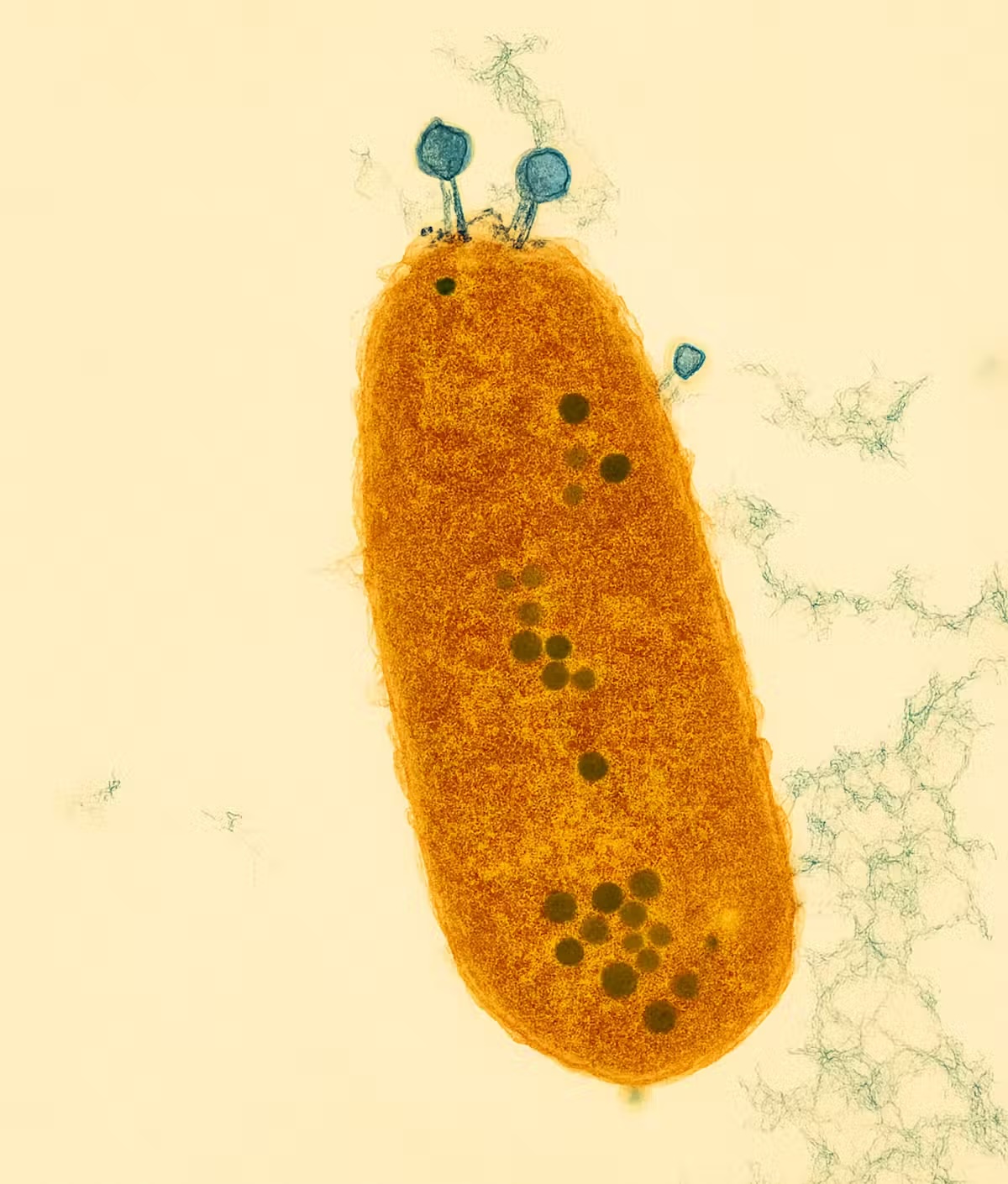
Some phages developed small mutations in the proteins they use to attach to the bacterial surface — subtle changes that helped them avoid triggering Kiwa’s detection system. Others took a different approach: they allowed themselves to be detected, but escaped the consequences.
These phages carried mutations in a viral protein that seems to be involved in how Kiwa shuts down the infection. We don’t yet know exactly how this works, but the result is clear: with just a few changes, the virus keeps replicating, even after Kiwa has been activated.
This evolutionary flexibility is part of what makes phages so powerful, and why they hold such promise in treating infections. But it also highlights a key challenge: to make phage therapy effective, we need to understand how these microbial battles play out.
Rules of engagement
If a bacterial strain carries a defense like Kiwa, not all phages will succeed against it. Some might be blocked entirely. But others, with just the right mutations, might slip through. That means choosing or engineering the right phage for the job is not just a matter of trial and error — it is a matter of knowing the rules of engagement.
Studying bacterial defense systems like Kiwa gives us a deeper understanding of those rules. It helps explain why some phages fail, why others succeed, and how we might design better phage therapies in the future. In time, we may be able to predict which bacterial defenses a given strain carries, and select phages that are naturally equipped — or artificially tuned — to overcome them.
That is the idea behind our growing phage collection project. We are gathering phages from across the U.K. and beyond, including from public submissions — dirty water is often a goldmine — and testing them to see which ones can overcome the defenses carried by dangerous bacteria. With over 600 types already catalogued, we are building a resource that could help guide future phage therapy, pairing the right phage with the right infection.
Kiwa is just one piece of the puzzle. Bacteria encode many such defense systems, each adding a layer of complexity — and opportunity — to this microbial arms race. Some detect viral DNA directly, others sense damage or stress, and some even coordinate responses with neighbouring cells. The more we learn, the more precisely we can intervene.
This is not a new war. Bacteria and phages have been locked in it for billions of years. But for the first time, we are starting to listen in. And if we learn how to navigate the strategies they have evolved, we might find new ways to treat the infections our antibiotics can no longer handle.
This edited article is republished from The Conversation under a Creative Commons license. Read the original article.